Cardiovascular
Research
At DELPHINIUM, cardiovascular research is a core pillar of our cardio-renal-metabolic focus. In close collaboration with the UMCG we conduct early-phase studies that combine high-quality clinical operations with advanced cardiovascular expertise. Studies are typically conducted in healthy volunteers, but, where justified and feasible, can also include selected patient populations with stable cardiovascular or cardiometabolic disease.
The Department of Cardiology at the University Medical Center Groningen (UMCG) has a long-standing history of high quality scientific output in international cardiovascular research. Overall focus area is the preservation of left ventricular function, with special interest in heart failure, atrial fibrillation and ischemic heart disease.
Collaboration UMCG
In collaboration with the department of Cardiology of the UMCG, we provide sponsors with direct access to leading cardiovascular investigators, advanced imaging and diagnostic facilities, and a large regional patient population.
This collaboration enables sponsors to:
- Execute first-in-human and other Phase I studies in a dedicated, GCP-compliant clinic directly connected to a university medical center
- Integrate sophisticated cardiovascular endpoints and imaging into early-phase protocols
- Plan a smooth transition from early-phase safety and PK/PD work to Phase II and beyond within the same academic ecosystem.
Cardiovascular safety and PD assessments
Within our cardiovascular studies, we can incorporate a range of assessments, depending on your compound’s profile and development questions.
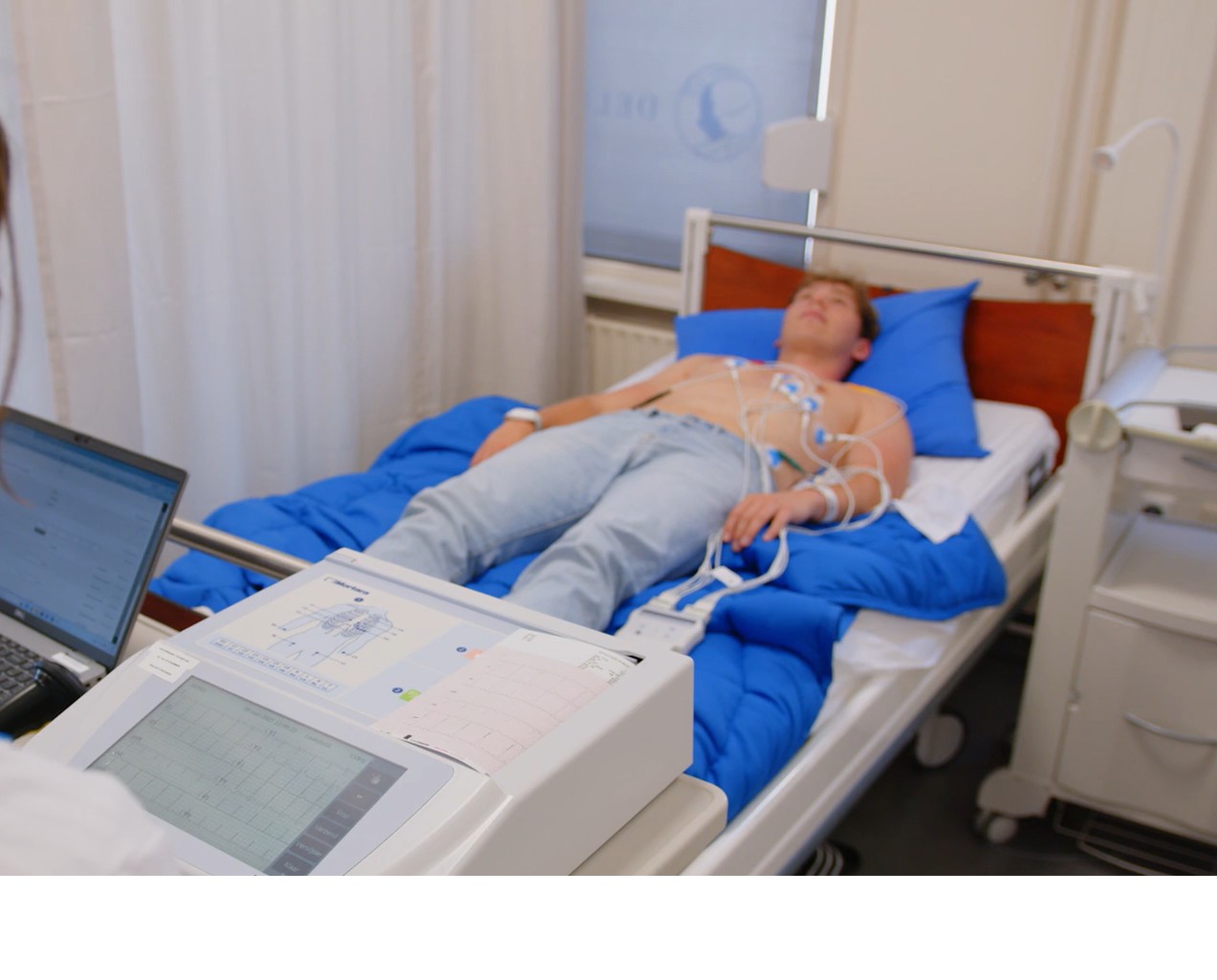
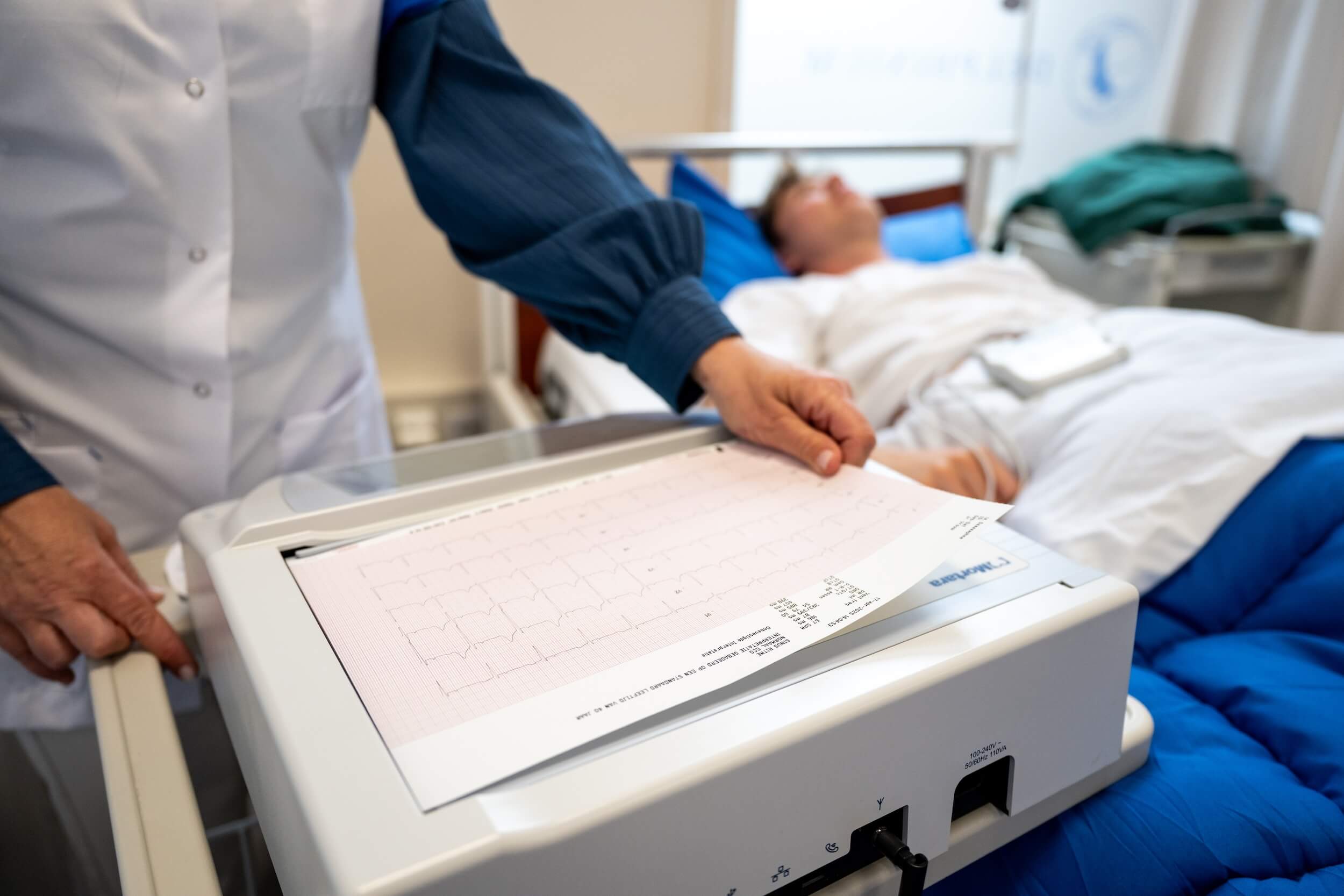
- 12-lead ECGs and continuous ECG monitoring,
- blood pressure and heart rate, including orthostatic measurements,
- routine clinical chemistry
- advanced cardiac biomarkers
- urinary and plasma proteomics, lipidomics and metabolomics
- Echo corelab facilities
Key Opinion Leader
Professor Peter van der Meer is a UMCG cardiologist and chair of Experimental Cardiology whose work spans basic discovery and patient care, with a strong focus on heart failure. His team uses cardiac stem-cell biology, human iPSC models, and engineered cardiac tissues to unravel disease mechanisms and identify therapeutic targets. He is an established investigator in early phase/FIH studies in cardiac patients and also leads and serves on steering and executive committees for multicenter heart-failure trials.
He is a recognized expert in cardiac amyloidosis—active within the Groningen Amyloidosis Center of Expertise (GrACE), a frequent presenter on the topic, a co-author on NEJM work demonstrating antibody-mediated ATTR clearance, and a contributor to ATTR trials such as ACT-EARLY and AMYLO-TAVR.

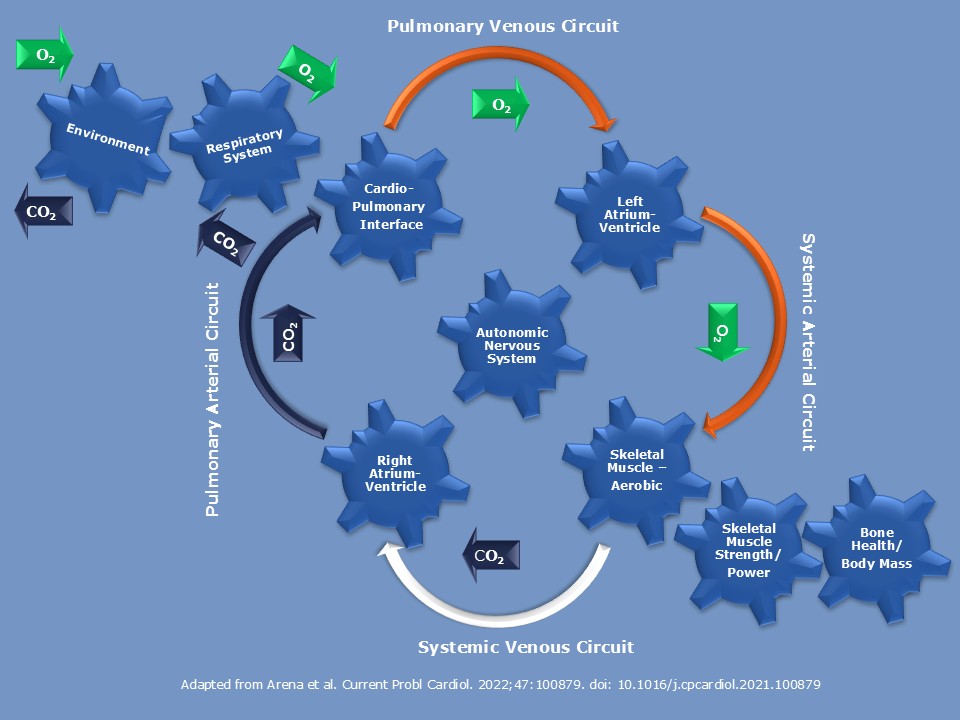
Cardiopulmonary Exercise Testing (CPET)
Cardiopulmonary exercise testing (CPET) is a standardized, non-invasive assessment of integrated cardiovascular, pulmonary, and muscular function during exercise. A key strength of CPET is its ability to quantify oxygen uptake and the ventilatory response under controlled conditions. Commonly reported parameters include peak VO₂ (a robust marker of aerobic capacity and prognosis in several cardiac conditions) and ventilatory efficiency, typically expressed as the VE/VCO₂ slope. Together, these outcomes help characterize limitations related to cardiac output, pulmonary perfusion/ventilation mismatch, peripheral oxygen extraction, and the abnormal ventilatory control.
CPET in clinical research
Within clinical research, CPET is used to:
- Phenotype disease severity and functional impairment (e.g., heart failure, pulmonary hypertension, congenital heart disease).
- Detect subtle physiological changes over time or after an intervention, even when resting imaging or biomarkers appear unchanged.
- Provide quantitative endpoints for clinical trials and mechanistic studies, including peak VO₂, ventilatory thresholds, oxygen pulse, heart-rate response and recovery, and exercise-induced symptoms.
DELPHINIUM, in collaboration with UMCG cardiology and dr. Ross Arena, has oversight over the complete data quality chain, ranging from qualified CPET lab personnel, equipment calibration and subject preparation to conducting the test, data capture and core lab processing and validation.
Supporting selecting functional assessment
DELPHINIUM also offers à-la-carte consultancy services to support sponsors in selecting the most appropriate functional assessment for their study (e.g. CPET or the 6-minute walk test). We advise on optimal test implementation, protocol alignment, and the selection of robust clinical trial endpoints that match the target population and study objectives. Sponsors are encouraged to engage with us early in development to ensure a scientifically sound and operationally feasible setup. This early alignment helps safeguard consistency and data quality from Phase 1 through Phase 3.
Key Opinion Leader
Dr. Arena received his B.S. in Human Performance from Southern Connecticut State University in 1993. He went on to receive his M.S. in Physical Therapy in 1997 and Ph.D. in Physiology in 2001 from the Medical College of Virginia/Virginia Commonwealth University. Dr. Arena is a globally recognized scholar in clinical exercise testing. He currently has over 1150 peer-reviewed publications, abstracts, and book chapters. Song et al (Front Cardiovasc Med. 2022 Aug 15:9:982351) analyzed the field of cardiopulmonary exercise testing from 2002-2021 and recognized “Ross Arena and Wasserman K being the most prolific and co-cited authors in the field”. Dr. Arena has overseen exercise testing core laboratory services for numerous clinical trials over the past 10 years.
For more information, please find here the paper: Expaning the concept of pharma-cise: A graphical primer for clinicians, researchers and industry of Dr. R. Arena.
